10 Small Submissions
No more than 10 Documents per submission
For years, Regulatory publishing was done by manual tasks. Publishers used to work for hours formatting documents, generating Agency-compliant PDFs, performing quality checks, compiling documents for submissions, and troubleshooting issues related to submissions.
Moreover, pharmaceutical companies are under immense pressure to submit error-free documents within stringent timelines. The inability to meet the timelines delay the product launch.
Although there is an increase in the adoption of Artificial Intelligence (AI) and automation in every industry, it is rare to find its impact in Regulatory affairs. Therefore, to overcome the challenges of manual, repetitive, and contextual document processes, organizations must step into the possibilities of the world using automation at the submission level and the whole process of compilation, validation, and document finalizing.
Automation offers a chance to enhance processes and workflow when preparing eCTD submissions in existing markets and expanding into newer markets. As enterprises consider automation for eCTD requests, it’s essential to consider the capabilities that will benefit and drive efficiency. Currently, some organizations have stepped into developing automation tools by using databases, but this is a time-consuming and manual activity.
The graph below demonstrates the number of days required for a company to file various applications using the manual process.
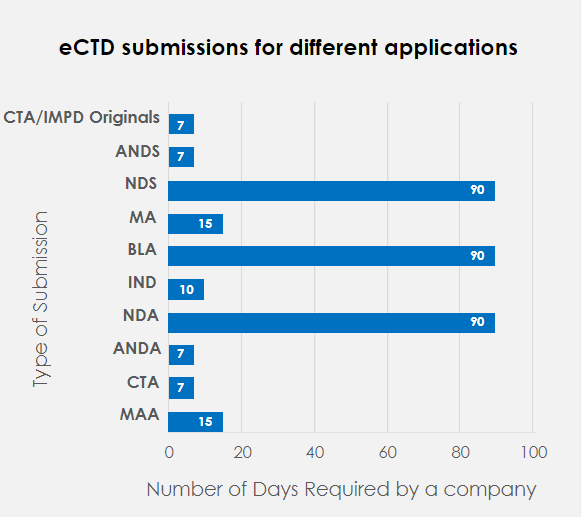
As we can see, it usually takes 90 days for companies to file a New Drug Application (NDA), and 15 days to file a Market Authorization Application (MAA). By automating a few repetitive steps, it is observed that an NDA Submission that usually takes 90 days can now be submitted within 30 days, approx. Thus, this could significantly reduce the publishing efforts by 57% every year and improve productivity by saving 60% time for publishers.
Hence, to eliminate time-consuming steps and drive efficiency in completing regular and repetitive tasks, the implementation of automated publishing tools for document level and submission level processes is the need of the hour.
Artificial intelligence can transform and have the power to revolutionize the end-to-end process of document management and submissions. With a massive experience of executing 100000+ global submissions – eCTD, NeeS and Paper formats – for large and small-to-medium BioPharma companies, Freyr has established the industry's first publishing automation innovation toolkit.
With the fresh and innovative method of evaluating day-to-day publishing activities from a simplified version, Freyr's Digital Publishing Automation is built using an array of RPA & NLP modules that automate document-level and submission-level publishing. It works on all Regulatory PDFs with the main header and sub-header, bookmarking and hyperlinking, keyword-based search, particular keyword highlights with colored font presentation, and internal and external hyperlinking between multiple documents. It works both on text-based PDFs and image-based PDFs. Publishing Automation tool is a REST API, cloud-based solution that is scalable and designed to work on all kinds of Regulatory PDFs from Health Authorities like the US FDA, EMA, HEALTH CANADA, SWISSMEDIC, SFDA, SAHPRA/MCCZA, TGA, and EAEU. To learn more about how you can automate your Regulatory publishing and submissions, Reach out to Freyr.