10 Small Submissions
No more than 10 Documents per submission
The Inbuilt eCTD validator supports all the regional and ICH validation criteria and can identify as many as 800+ error scenarios. It enables users to validate their submissions for various Health Authorities and their eCTD requirements. The eCTD software’s inbuilt validator simplifies the submission steps and increases overall efficiency by providing a comprehensive technical validation report.
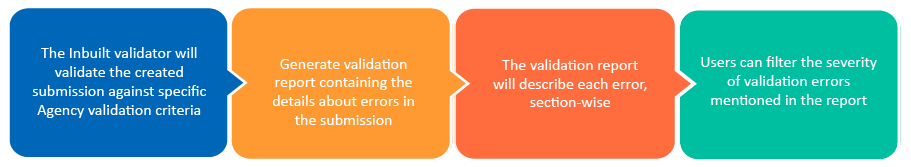
The inbuilt eCTD Validator in Freyr SUBMIT PRO ensures that your submissions comply with the latest eCTD validation criteria set by the agencies for hassle-free submissions. Freyr’s eCTD Validator generates a detailed technical validation report with errors and strings them to the technical validation rules to understand the various technical validation criteria such as FDA eCTD validation criteria, EU eCTD validation criteria, etc., taken to resolve those errors.