10 Small Submissions
No more than 10 Documents per submission

The Electronic Common Technical Document (eCTD) has become the golden standard for drug regulatory submissions worldwide, and with the advent of eCTD 4.0 in 2022, a new chapter of innovation began. While this latest version boasts remarkable benefits like heightened efficiency and precision, it also ushers in unique challenges for countries transitioning to this cutting-edge format. In this blog, we'll embark on a journey to uncover these challenges, as the world navigates the transformative waters of eCTD 4.0.
Transitioning to eCTD 4.0 may present its share of challenges, but they are conquerable with dedication and determination from countries. While significant investment and effort will be required, the rewards that eCTD 4.0 brings are equally substantial, making the endeavor truly worthwhile.
Amidst these challenges, countries can take proactive steps to pave a smoother path toward eCTD 4.0 adoption. By following purposeful efforts and strategic approaches, they can unlock the full potential of this innovative standard and usher in a new era of Regulatory excellence.
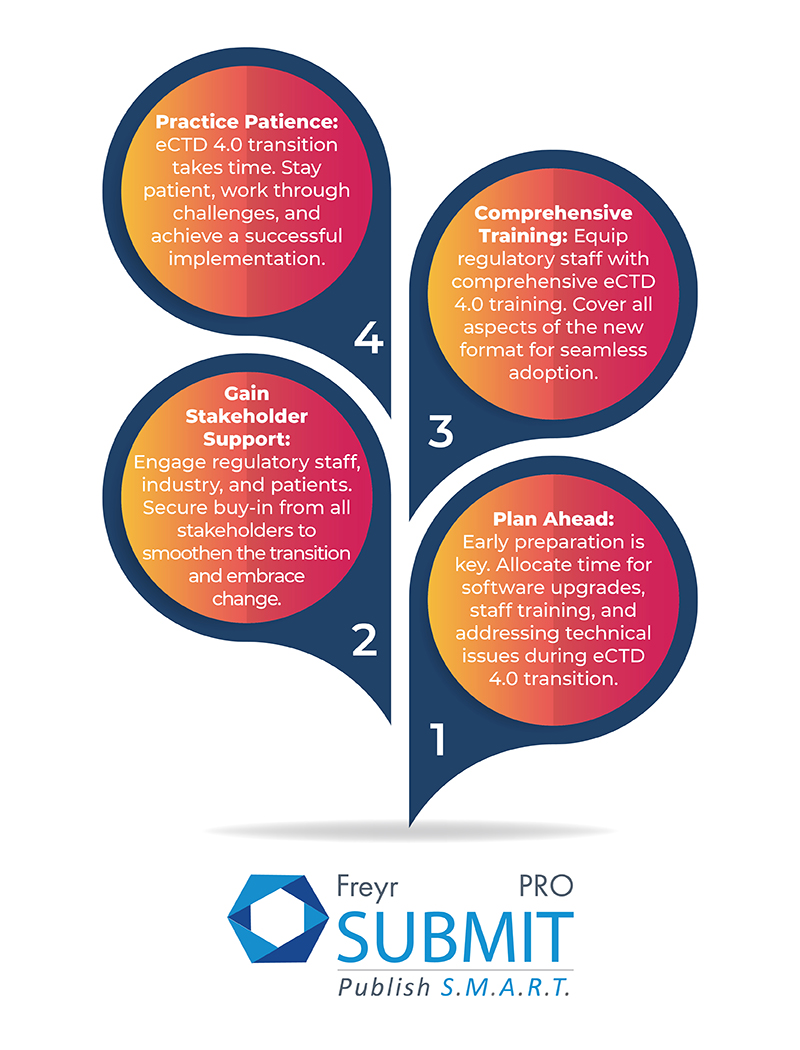
Transitioning to eCTD 4.0 might seem like a daunting task, but fear not! With the right strategy and guidance, countries can conquer this challenge and unlock the full potential of eCTD 4.0's wonders. Partnering with vendors like Freyr, who have a reach in about 20 countries around the world and are well-versed in Regulatory publishing, is the key to success for life sciences organizations. At Freyr, we take pride in driving innovation through technology, and our Regulatory submission and publishing software, Freyr SUBMIT PRO. Got quick queries on eCTD submissions? Contact us today and pave the way for a seamless Regulatory future!