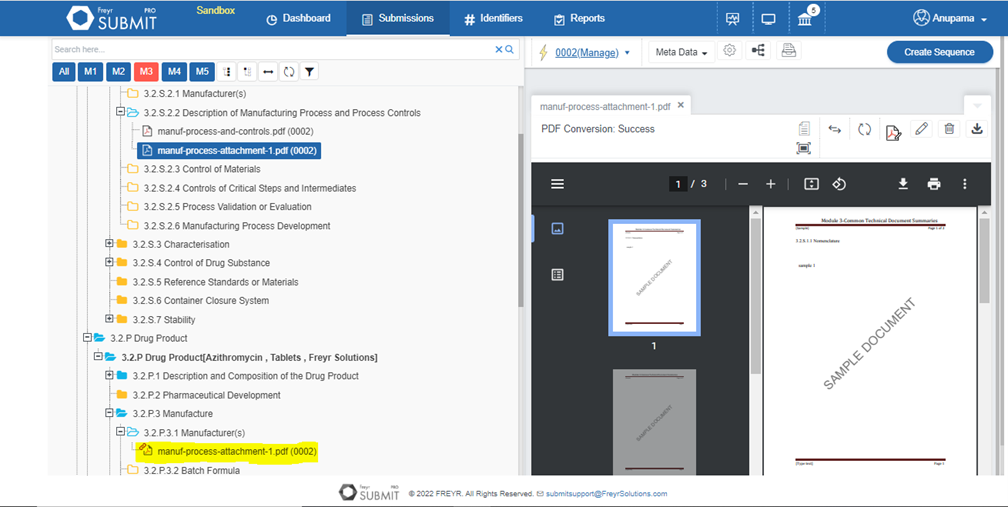
10 Small Submissions
No more than 10 Documents per submission
Ensure comprehensive validation report generation of submissions for various HAs & their eCTD requirements by identifying most errors.
Learn More
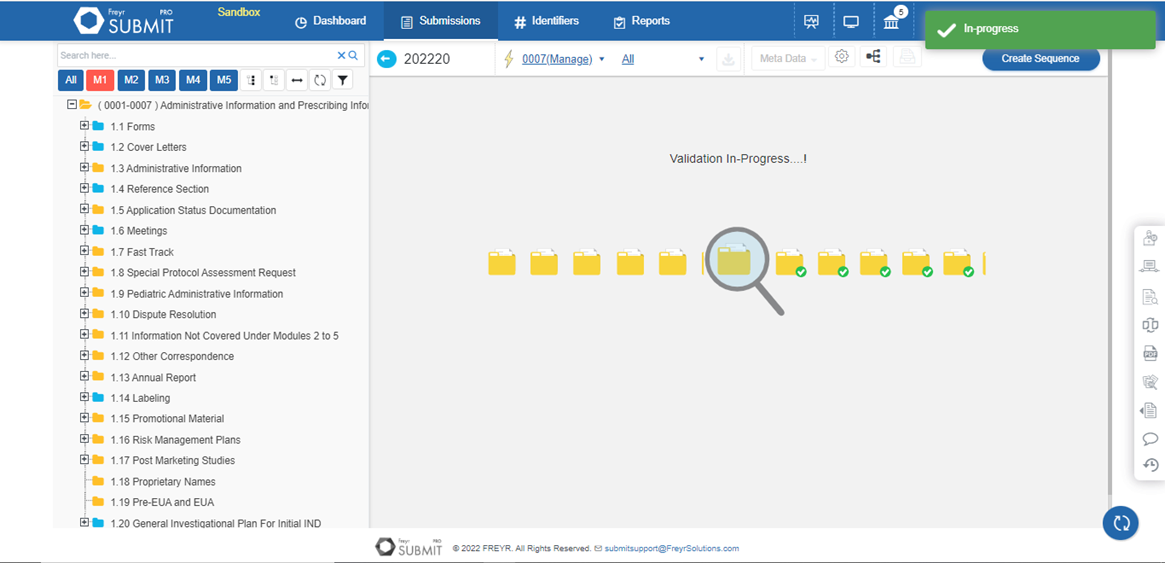

Eliminate the risk of missing submission deadlines with Freyr SUBMIT PRO. Its intelligent reporting system can track the number of submissions, review them, and configure alerts collaboratively. This allows one-stop tracking of all compliance-related processes and thus enables informed and quick decision making.
Learn More
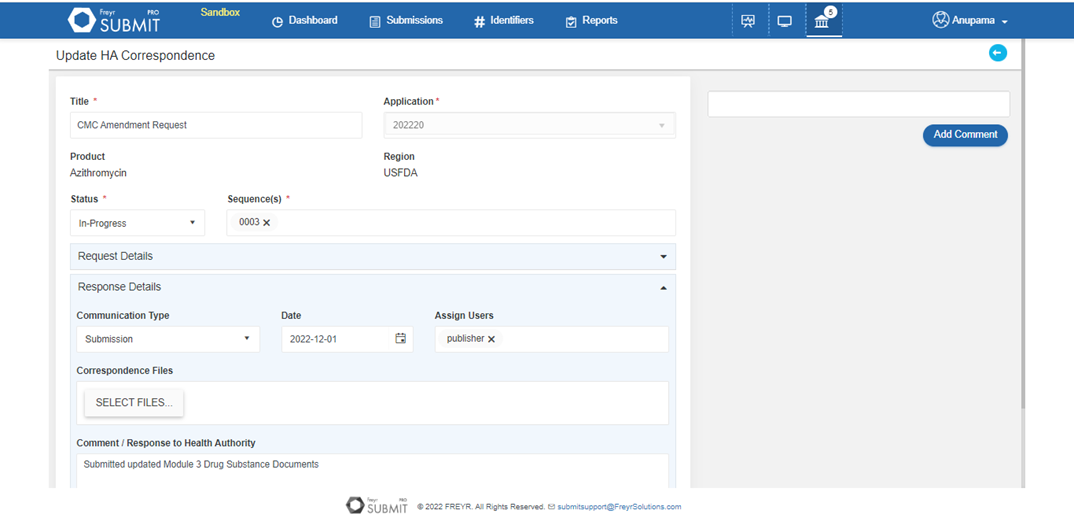
Track all Health Authorities’ queries centrally for each product with Freyr SUBMIT PRO and ensure speedy approval by on-time communication with HA.

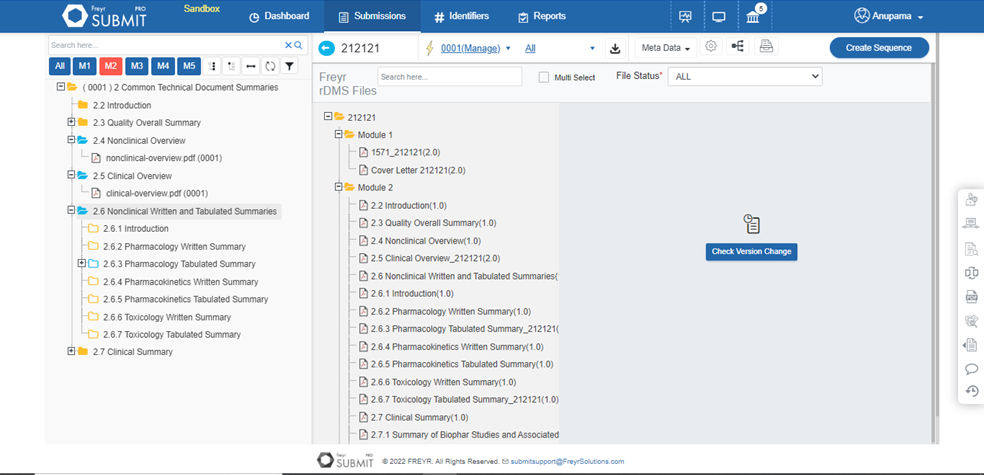
Built for flexibility & security, Freyr SUBMIT PRO facilitates seamless integration with existing rDMS to ensure confidential data transfers to Freyr SUBMIT PRO eCTD tool in the most secure manner.
Learn More

Create easy-to-review documents for quick product approvals & adhere to submission deadlines. Freyr SUBMIT PRO offers a simplified environment for the users to collaborate on the same document/submission, facilitating reviews and meeting agency deadlines successfully.
Learn More
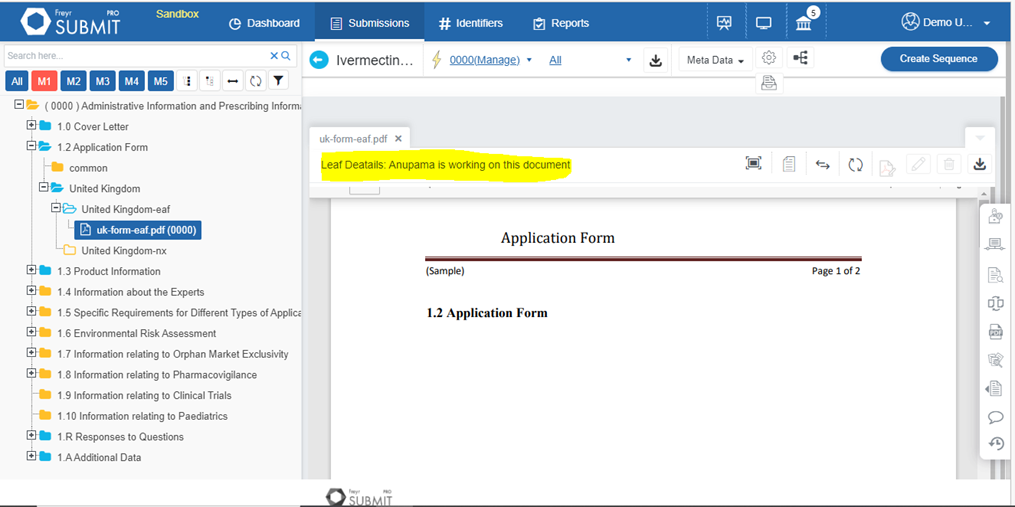

Eliminate submission errors with Freyr SUBMIT PRO. It allows the user to view submissions, including legacy submissions in a cumulative table of content, Regulatory, and study views.
Learn More
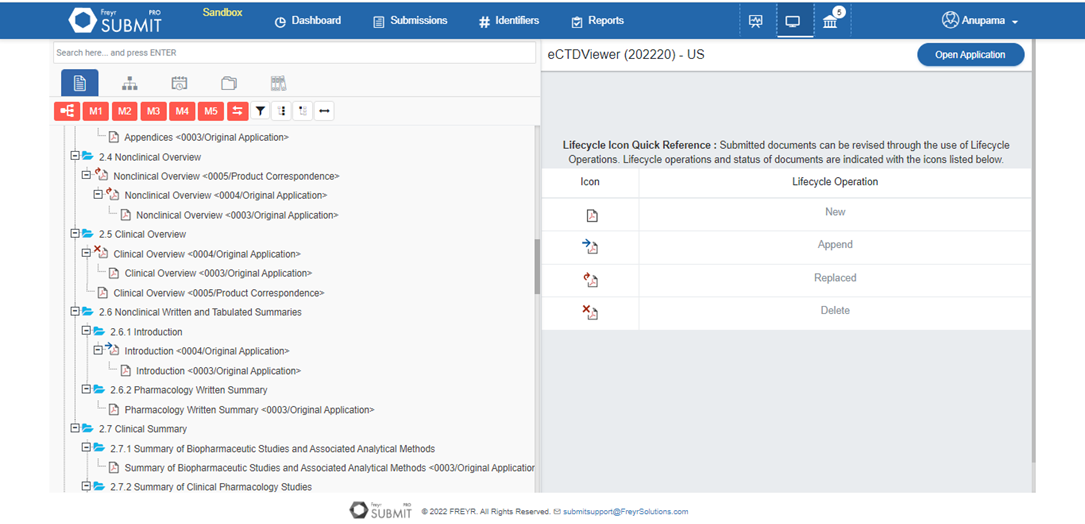

Make your eCTD submissions efficient, secure, and more manageable with Freyr SUBMIT PRO’s inbuilt PDF manager. With features like bookmarks, internal & external hyperlinks, and specified access control settings, Freyr SUBMIT PRO helps users maintain documents coherently.
Learn More
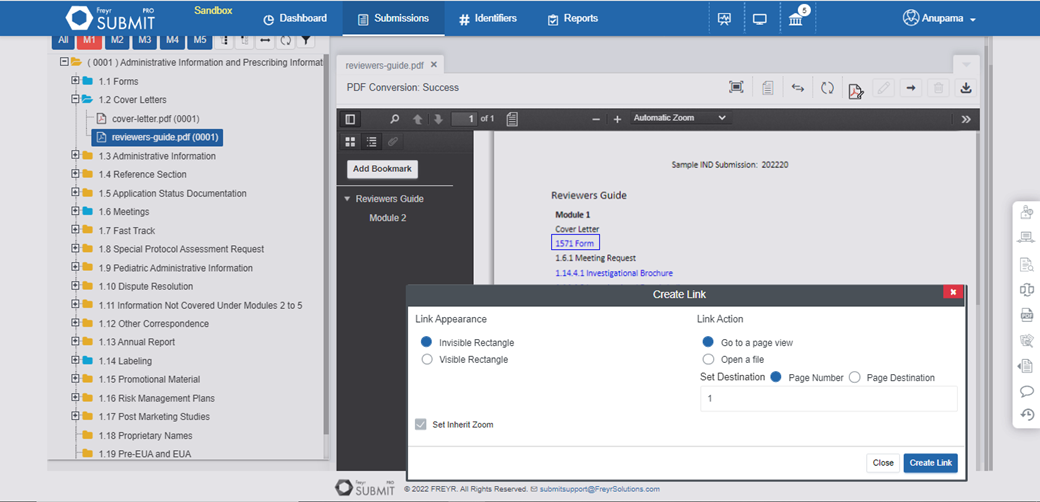

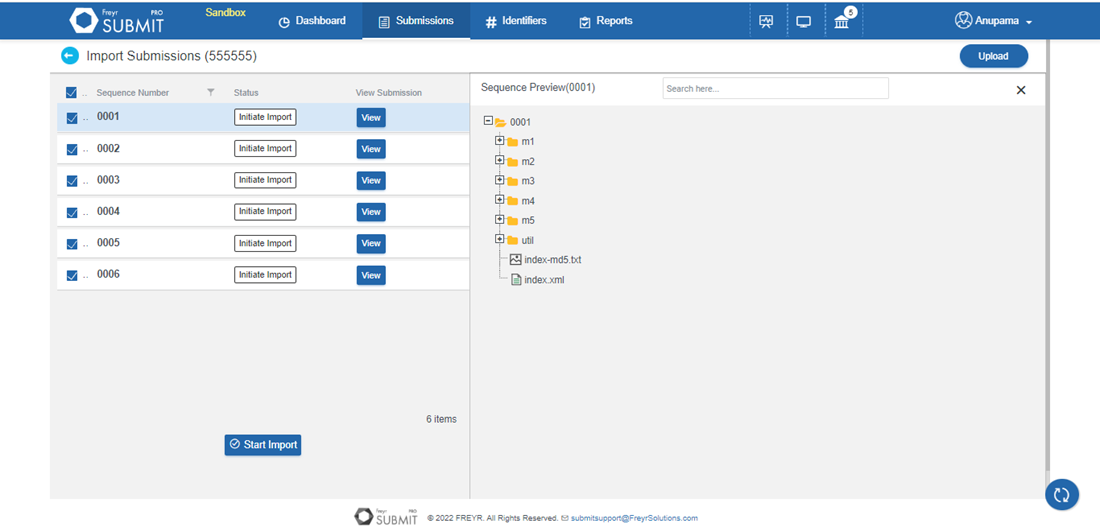
Migrate any pre-owned dossier from other platforms to Freyr SUBMIT PRO with ease. Enable better life cycle management with the import utility of Freyr SUBMIT PRO.
Learn More

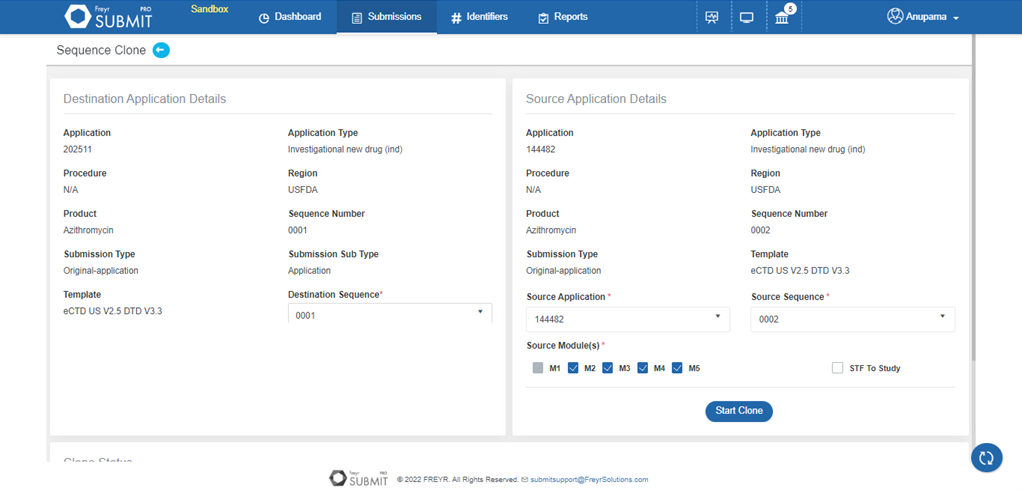
Freyr SUBMIT PRO offers a cloning option except for eCTD Modules that are not region-specific (Modules 2 to 5). Thus, reducing cost per submission and saving time.
Learn More

Freyr SUBMIT PRO offers cross-reference feature wherein applicants can mention the path of the physical files instead of duplicating or cloning.
Learn More